Harnessing Fluorosilicic Acid in Aluminum Electrolysis: A Sustainable Approach
The aluminum industry is constantly evolving, driven by the demand for efficiency, cost reduction, and environmental sustainability. One of the key components in aluminum production is fluorosilicic acid (H2SiF6), a byproduct of phosphate fertilizer and hydrogen fluoride manufacturing. This versatile chemical plays a crucial role in aluminum electrolysis, particularly in the production of aluminum fluoride (AlF3) and synthetic cryolite (Na3AlF6), essential materials for the Hall-Héroult process.
In this article, we explore how fluorosilicic acid is utilized in aluminum electrolysis, its benefits over traditional raw materials, and why it represents a cost-effective and eco-friendly solution for modern smelters.
1. The Role of Fluorides in Aluminum Electrolysis
Aluminum is produced through the Hall-Héroult process, where alumina (Al2O3) is dissolved in a molten cryolite (Na3AlF6) bath and electrolyzed at high temperatures (~950°C). The process requires:
● Cryolite (Na3AlF6) – Lowers the melting point of alumina.
● Aluminum Fluoride (AlF3) – Adjusts bath chemistry, improving conductivity and reducing energy consumption.
Traditionally, these fluorides are sourced from mined fluorspar (CaF2) or synthetic production. However, fluorosilicic acid offers a sustainable alternative, especially as it is a byproduct of other industrial processes.
2. How Fluorosilicic Acid is Converted into Aluminum Fluoride and Cryolite
Fluorosilicic acid is a low-cost, high-efficiency precursor for manufacturing aluminum fluorides. The conversion process involves several key steps:
A. Production of Aluminum Fluoride (AlF3)
① Neutralization with Alumina or Aluminum Hydroxide
Fluorosilicic acid reacts with alumina (Al2O3) or aluminum hydroxide (Al(OH)3): 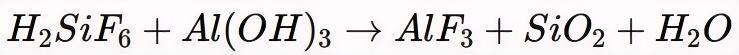
The silica (SiO2) byproduct is filtered out, leaving a purified AlF3 solution.
② Crystallization & Drying
The solution is evaporated, crystallized, and dried to produce anhydrous AlF₃, a critical additive for aluminum smelting.
B. Production of Synthetic Cryolite (Na3AlF6)
① Reaction with Sodium Hydroxide or Soda Ash
Fluorosilicic acid is neutralized with sodium hydroxide (NaOH) or sodium carbonate (Na2CO3):
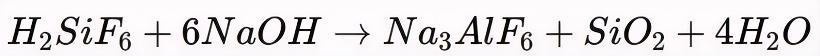
The resulting cryolite is filtered, washed, and dried for smelter use.
② High-Purity Cryolite for Smelting
Synthetic cryolite from H₂SiF₆ has consistent quality, unlike natural cryolite, which may contain impurities.
3. Advantages of Using Fluorosilicic Acid in Aluminum Production
■ Cost Efficiency
Fluorosilicic acid is a low-cost byproduct of phosphate fertilizer production, reducing reliance on expensive fluorspar.
■ Environmental Sustainability
a. Recycling H2SiF6 prevents its release as a pollutant, aligning with circular economy principles.
b. Reduces the need for mining fluorspar, lowering the carbon footprint.
■ Improved Smelting Performance
a. High-purity AlF₃ and cryolite enhance bath conductivity, reducing energy consumption.
b. Stabilizes the electrolyte, minimizing anode effects and improving cell life.
■ Supply Chain Reliability
Unlike fluorspar, which is subject to geopolitical supply risks, fluorosilicic acid is widely available from fertilizer plants.
4. Industry Trends & Future Outlook
With aluminum demand rising (especially in EVs and renewable energy), smelters are seeking sustainable and cost-effective fluoride sources. Fluorosilicic acid is gaining traction due to:
● Government regulations promoting waste utilization.
● Energy efficiency requirements in smelting.
● Corporate ESG (Environmental, Social, Governance) goals favoring recycled materials.
Companies investing in fluorosilicic acid-based fluorides are positioning themselves as innovative and eco-conscious suppliers in the aluminum industry.
5. Why Partner with Us?
At Foshan Nanhai Shuangfu Chemical Co. Ltd, we specialize in high-purity fluorosilicic acid for aluminum smelting. Our products ensure:
★ Lower production costs compared to traditional fluorspar-based fluorides.
★ Consistent quality for optimal smelting performance.
★ Sustainable sourcing, reducing environmental impact.
Contact us today to learn how our fluorosilicic acid solutions can enhance your aluminum production efficiency!
Fluorosilicic acid is revolutionizing aluminum electrolysis by providing a cost-effective, sustainable, and high-performance alternative to conventional fluoride sources. As the industry moves toward greener practices, adopting H₂SiF₆-based fluorides is a strategic advantage for smelters worldwide.
By leveraging this innovative approach, your company can reduce costs, improve efficiency, and meet sustainability targets—making fluorosilicic acid a key player in the future of aluminum production.
Explore our fluorosilicic acid products today and elevate your smelting process!




